eye Insights Issue 02: Fueling a Revolution in Retinal Care
 How biomedical breakthroughs in anti-VEGF research have changed how we diagnose, treat, and save vision for patients with retinal disease.
How biomedical breakthroughs in anti-VEGF research have changed how we diagnose, treat, and save vision for patients with retinal disease.
|
Inside:
- 2014 António Champalimaud Vision Award
- AMD Treatment Guidelines
- Fueling a Revolution in Retinal Care
- Novel Strategies Set the Stage for Next Generation Therapies
Editor-in-Chief: Joan W. Miller, MD, FARVO
Managing Editor: Matthew F. Gardiner, MD
Communications Director: Suzanne Ward
Scientific Communications Manager: Wendy Chao, PhD
Clinical Advisory Group: Carolyn E. Kloek, MD, Deeba Husain, MD, Ankoor S. Shah, MD, PhD, Angela V. Turalba, MD
Browse all issues >> | Email: eyeinsights@meei.harvard.edu
| eye Insights 2 PDF | 2.37 MB |
2014 Antonio Champalimaud Vision Award
2014 António Champalimaud Laureates: Joan W. Miller, MD, FARVO, Evangelos S. Gragoudas, MD, Patricia A. D’Amore, PhD, MBA, FARVO, Lloyd Paul Aiello, MD, PhD, George L. King, MD, Anthony P. Adamis, MD; and Napoleone Ferrara, MD
Dear Colleagues,
In September, several Mass. Eye and Ear/Harvard Medical School (HMS) Department of Ophthalmology colleagues and I were among seven researchers honored with the 2014 António Champalimaud Vision Award, the highest distinction in ophthalmology and visual science, for our role in the development of anti-angiogenic therapy for retinal disease. This series of translational breakthroughs led to a new class of ophthalmic anti-VEGF drugs, which have revolutionized patient care for neovascular age-related macular degeneration (AMD), diabetic macular edema and macular edema following retinal vein occlusion. Prior to these developments, neovascular AMD caused 90 percent of AMD-related blindness. With today’s treatments, vision loss can now be avoided in many patients. In fact, up to one-third of neovascular AMD patients treated with anti-VEGF drugs now experience significant improvements in visual acuity.
These advancements dramatically improved the outlook for many patients. However, our work continues as we strive to better understand the pathogenesis of AMD and to develop more patient-friendly treatments that aim to prevent retinal disease and preserve vision function. To power our efforts, in 2011 our department underwent a significant milestone when Mass. Eye and Ear joined forces with Schepens Eye Research Institute. This exciting union integrated the efforts of our 100+ faculty and significantly enhanced our bench-to-bedside bandwidth by blending our unique strengths in bench and translational research. Today, collaborations abound across the department, leveraging advances in biotechnology and human genetics that keep our efforts at the forefront of cutting-edge retinal research.
We hope you enjoy this issue of Eye Insights, which explores how far we’ve come over the last decade in bringing sight-saving, anti-VEGF treatments to patients with AMD, diabetic macular edema, and retinal vein occlusion, and highlights new efforts that are underway. As always, we hope you find Eye Insights to be a useful tool in your patient armamentarium.
Sincerely,
Joan W. Miller, MD, FARVO
Henry Willard Williams Professor of Ophthalmology
Chair, Harvard Medical School
Department of Ophthalmology
Chief of Ophthalmology
Massachusetts Eye and Ear and
Massachusetts General Hospital
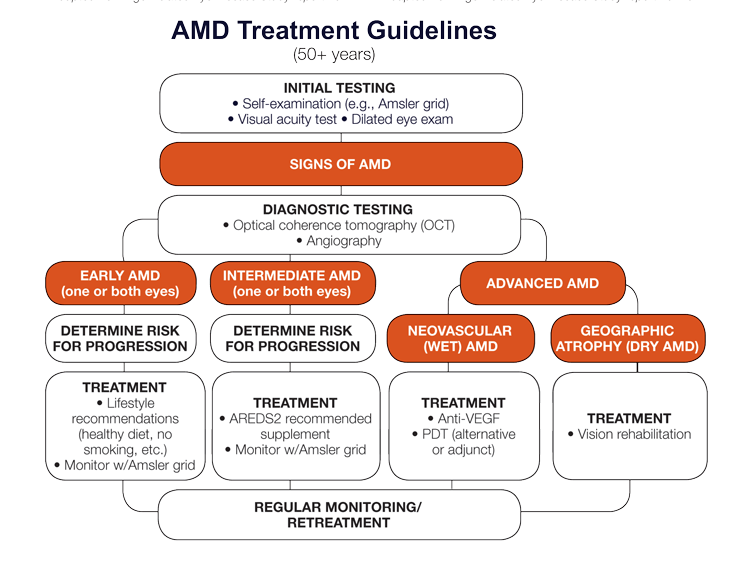
AMD Treatment Guidelines
AMD is the leading cause of vision loss in developed countries, and accounts for 8.7% of visual impairment worldwide. AMD is caused by a combination of genetic and environmental factors with age being the greatest risk factor. In the United States, AMD affects 7 percent of people age 60-69 and 35 percent of people age 80 and older (National Institutes of Health). AMD occurs in two forms: dry (nonexudative) and wet (exudative or neovascular). Ninety percent of all people with AMD have the dry type, which includes the early and intermediate stages of AMD, as well as the advanced form known as geographic atrophy. The wet form affects 10 percent of all people with AMD, and accounts for 90 percent of legal blindness from the disease.
Estimating Risk of AMD Progression
The Age-Related Eye Disease Study (AREDS) 9-step severity scale and the simplified 5-step severity scale use drusen size and pigmentary abnormalities to determine a risk score upon clinical examination.
| AREDS Risk Factor Scoring Systemb |
| +1 For each eye with large drusen |
| +1 For each eye with pigment abnormalities |
| +1 If neither eye has large drusen, but both eyes have intermediate drusen |
| +2 For the eye that has neovascular AMD |
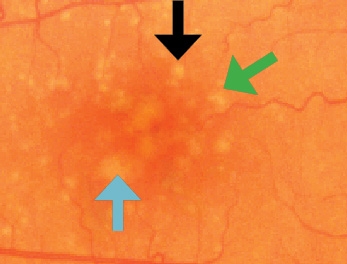
Drusen Classificationa
Small: <63 μm
Intermediate (black arrow): 63-124 μm
Large (green arrow): 125*-249 μm
Very Large (blue arrow): >250 μm
* 125 μm is roughly the width of a retinal vein
where it crosses the optic disc.
|
AREDS Simplified Severity Scaleb |
0 | 1 | 2 | 3 | 4 |
| 5-year rates of progression to advanced AMD | 0.5% | 3% | 12% | 25% | 50% |
aAdapted from Age-Related Eye Disease Study report no. 17 bAdapted from Age-Related Eye Disease Study report no. 18
Fueling a Revolution in Retinal Care
 In the 1990s, the 2014 Champalimaud Award Laureates worked in parallel and in collaboration to identify vascular endothelial growth factor (VEGF) as the major trigger for angiogenesis in the eye. In 1993, they showed that the human retina synthesizes VEGF, and subsequently demonstrated that VEGF expression is induced in low-oxygen conditions. In 1994, the team correlated VEGF with ocular angiogenesis in primates (American Journal of Pathology), which was the first in vivo demonstration of VEGF’s role in ocular neovascularization. That same year, the team published two separate studies (New England Journal of Medicine, American Journal of Ophthalmology) both demonstrating increased VEGF in the vitreous of patients with proliferative diabetic retinopathy. A subsequent study describing a mouse model of retinopathy of prematurity and other oxygen-induced retinal disorders became the most-cited article in the journal Investigative Ophthalmology and Visual Science. In a series of studies published between 1995 and 1996, the investigators demonstrated that VEGF inhibitors could block ocular neovascularization in preclinical models. This cumulative work provided the scientific foundation for the development of anti-VEGF therapies – now the gold standard for treating neovascular AMD, diabetic macular edema and retinal vein occlusion. VEGF inhibitors hold potential for a growing list of indications, including neovascular glaucoma and retinopathy of prematurity.
In the 1990s, the 2014 Champalimaud Award Laureates worked in parallel and in collaboration to identify vascular endothelial growth factor (VEGF) as the major trigger for angiogenesis in the eye. In 1993, they showed that the human retina synthesizes VEGF, and subsequently demonstrated that VEGF expression is induced in low-oxygen conditions. In 1994, the team correlated VEGF with ocular angiogenesis in primates (American Journal of Pathology), which was the first in vivo demonstration of VEGF’s role in ocular neovascularization. That same year, the team published two separate studies (New England Journal of Medicine, American Journal of Ophthalmology) both demonstrating increased VEGF in the vitreous of patients with proliferative diabetic retinopathy. A subsequent study describing a mouse model of retinopathy of prematurity and other oxygen-induced retinal disorders became the most-cited article in the journal Investigative Ophthalmology and Visual Science. In a series of studies published between 1995 and 1996, the investigators demonstrated that VEGF inhibitors could block ocular neovascularization in preclinical models. This cumulative work provided the scientific foundation for the development of anti-VEGF therapies – now the gold standard for treating neovascular AMD, diabetic macular edema and retinal vein occlusion. VEGF inhibitors hold potential for a growing list of indications, including neovascular glaucoma and retinopathy of prematurity.
|
2000 Visudyne® |
As the first treatment for neovascular AMD, photodynamic therapy with verteporfin (Visudyne) is approved by the FDA and international drug regulatory agencies, opening the pharmacologic era of retinal disease therapy. |
|
2004 Macugen® |
Following results from a large multicenter clinical trial published in New England Journal of Medicine, pegaptanib (Macugen) becomes the first FDA-approved anti-VEGF therapy for |
|
2004 Avastin® |
Bevacizumab (Avastin) – originally FDA-approved 2004 as an anti-VEGF drug for cancer – is successfully administered off-label via intravitreal injection to treat neovascular AMD. By early 2006, Avastin is widely used off-label for neovascular AMD. |
|
2006 Lucentis® |
Ranibizumab (Lucentis) receives FDA approval for the treatment of AMD. This was hailed as one of the top ten breakthroughs of 2006 by the journal Science. |
|
2010 Lucentis® |
Ranibizumab receives FDA approval for the treatment of macula edema following retinal vein occlusion. |
|
2011 Avastin® and Lucentis® |
Bevacizumab (Avastin) is shown to have similar efficacy as ranibizumab when administered according to the same schedule in the Comparison of AMD Treatments Trials (CATT) by the National Institutes of Health (NIH). Bevacizumab is a cost-effective alternative to ranibizumab and the most widely used off-label treatment today for neovascular AMD. Bevacizumab is also used to treat diabetic macular edema and retinal vein occlusion. A study also estimated that two years of Lucentis treatment reduces visual impairment in neovascular AMD by 37 percent and legal blindness by 72 percent. |
|
2011 Eyelea® |
The most recent FDA- approved anti-VEGF therapy, aflibercept (Eylea) requires fewer intraocular injections than other anti-VEGF therapies and has become the predominant FDA-approved therapy for treating neovascular AMD. |
|
2012 Lucentis® |
Ranibizumab receives FDA approval for the treatment of diabetic macular edema. |
Should Genetic Testing Guide AREDS Recommendations?
 Two studies provide different recommendations regarding whether genetic testing should be used to guide Age-Related Eye Disease Study (AREDS) supplemental recommendations. “This is one of the hottest debates at present regarding treatment for AMD. At some level, both studies may be right,” noted Joan W. Miller, MD, FARVO, Chair of the HMS Department of Ophthalmology and Chief of Ophthalmology at Mass. Eye and Ear and Mass General Hospital. “AREDS2 recommendations are reasonable to follow at present. When we have a new and better treatment for early AMD, it is quite possible that genetic testing will be used to select the “best” treatment for an individual."
Two studies provide different recommendations regarding whether genetic testing should be used to guide Age-Related Eye Disease Study (AREDS) supplemental recommendations. “This is one of the hottest debates at present regarding treatment for AMD. At some level, both studies may be right,” noted Joan W. Miller, MD, FARVO, Chair of the HMS Department of Ophthalmology and Chief of Ophthalmology at Mass. Eye and Ear and Mass General Hospital. “AREDS2 recommendations are reasonable to follow at present. When we have a new and better treatment for early AMD, it is quite possible that genetic testing will be used to select the “best” treatment for an individual."
Improving Anti-VEGF Treatment for AMD
Developing more patient-friendly, less burdensome treatments for patients with neovascular disease is an important goal of ongoing research efforts. Approved by the FDA in 2011 for treating neovascular AMD, aflibercept (Eylea) has an alternative mechanism of VEGF blockade that has been shown to have higher binding affinities compared to ranibizumab and bevacizumab. Mass. Eye and Ear investigators conducted a study exploring the short-term visual and anatomic outcomes of patients with refractory or recurrent neovascular AMD who were converted from bevacizumab and/or ranibizumab to aflibercept. They also examined whether aflibercept conversion could extend the injection intervals to decrease injection burden. The data suggest that converting patients with chronic neovascular AMD to aflibercept resulted in stabilized vision and improved anatomic outcomes, allowing a less frequent dosing schedule of every 8 weeks after 3 initial monthly injections. Further studies are warranted to determine whether the conversion benefits to aflibercept are sustained. Looking ahead, pharmacogenomic technology may play a role in guiding treatment for personalized medicine. Researchers are working toward developing better methods of phenotyping using improved imaging techniques, metabolomics, and genetic animal models.
Multiple Treatment Options
Given the multiple treatment options for neovascular AMD, the selection of medication and treatment modality depends on the type of lesion, as well as the patient’s systemic health, social circumstances, and economic considerations. Having multiple options has been particularly helpful in treating difficult cases. For example, switching from one anti-VEGF medication to another has been shown to be effective in recurrent and refractory choroidal neovascularization. Photodynamic therapy (PDT) with verteporfin is still an effective method of treating refractory cases. Moreover, a multicenter, randomized controlled trial has shown that combination therapy (PDT with an anti-VEGF agent) may be an effective approach for treating polypoidal choroidal vasculopathy (PCV), while a retrospective review demonstrated promising results using triple therapy (PDT with an anti-VEGF agent plus a steroid) for PCV.
Clinical Resources
- Miller JW. Age-related macular degeneration revisited–piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol 2013 Jan;155(1):1-35.e13. doi: 10.1016/j.ajo.2012.10.018.
- Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, Vavvas DG, Miller JW, Kim IK. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 2013;156:29-35.e22.
- Yonekawa Y, Kim IK. Clinical characteristics and current treatment of age-related macular degeneration. Cold Spring Harb Perspect Med 2014 Oct 3. pii: a017178. doi: 0.1101/cshperspect.a017178.
Novel Strategies Set the Stage for Next Generation Therapies
While significant progress has been achieved in treating retinal diseases, researchers at Mass. Eye and Ear/Schepens Eye Research Institute remain deeply committed to improving current therapies, refining diagnostic tools, and developing new therapies that leverage advances in biotechnology and human genetics. Collaborations are ongoing throughout the department’s Centers of Excellence and Institutes. Some current avenues of study and research include:
 To target new disease pathways, researchers in the AMD Center of Excellence are studying genetic and epidemiological risk factors that make some people more susceptible to AMD. They are also trying to improve their understanding of early disease progression using dark adaptation, novel imaging devices and metabolomics. Researchers are also developing neuroprotective agents in combination with anti-VEGF therapies to prevent photoreceptor cell death – the ultimate cause of vision loss in AMD.
To target new disease pathways, researchers in the AMD Center of Excellence are studying genetic and epidemiological risk factors that make some people more susceptible to AMD. They are also trying to improve their understanding of early disease progression using dark adaptation, novel imaging devices and metabolomics. Researchers are also developing neuroprotective agents in combination with anti-VEGF therapies to prevent photoreceptor cell death – the ultimate cause of vision loss in AMD.
 In 2013, the Ocular Genomics Institute (OGI) published the most thorough description of gene expression in the human retina to date (BMC Genomics), which is crucial to understanding how diseases of the eye develop and lead to vision loss. This is a valuable resource for the vision research community, and the data are available via the OGI website. OGI researchers also demonstrated that the complement system, which is part of the immune system, plays a critical role in the early stage of an inherited macular degeneration (Human Molecular Genetics). Drugs that inhibit specific complement system activities are being clinically tested
In 2013, the Ocular Genomics Institute (OGI) published the most thorough description of gene expression in the human retina to date (BMC Genomics), which is crucial to understanding how diseases of the eye develop and lead to vision loss. This is a valuable resource for the vision research community, and the data are available via the OGI website. OGI researchers also demonstrated that the complement system, which is part of the immune system, plays a critical role in the early stage of an inherited macular degeneration (Human Molecular Genetics). Drugs that inhibit specific complement system activities are being clinically tested
as treatments for AMD.
 Members of the Ocular Regenerative Medicine Institute (ORMI) are participating in a Phase I/II clinical trial to evaluate the safety of human embryonic stem cell (hESC)-derived retinal pigment epithelial (RPE) cells for dry AMD. Mass. Eye and Ear is serving as a clinical trial site for the U.S. and European study, which is being conducted by Advanced Cell Technology, Inc., a leader in the field of regenerative medicine. ORMI members are also developing engineered biomaterials that may be used to deliver neuroprotective agents or stem cells to the retina with plans to conduct a first-in-man restorative stem cell trial in early 2015.
Members of the Ocular Regenerative Medicine Institute (ORMI) are participating in a Phase I/II clinical trial to evaluate the safety of human embryonic stem cell (hESC)-derived retinal pigment epithelial (RPE) cells for dry AMD. Mass. Eye and Ear is serving as a clinical trial site for the U.S. and European study, which is being conducted by Advanced Cell Technology, Inc., a leader in the field of regenerative medicine. ORMI members are also developing engineered biomaterials that may be used to deliver neuroprotective agents or stem cells to the retina with plans to conduct a first-in-man restorative stem cell trial in early 2015.
 Members of the Mobility Enhancement and Vision Rehabilitation Center of Excellence are working to find creative ways to help patients with impaired vision achieve greater independence and mobility, and a better quality of life. One vision-enhancing technology is SuperVision+, a free smart phone magnifier app available for iOS and Android platforms. In addition to magnifying small print (i.e., medication bottles and restaurant menus), the app has a unique image-stabilization feature that “locks” shaky images caused by hand tremors. Another tech-savvy application is utilizing video games to help patients develop navigation skills (way-finding) and improve their sense of independence. Center members are also involved in research addressing contrast sensitivity, fundus-related perimetry, and visual hallucinations in patients with vision loss, as well as development of a retinal prosthesis.
Members of the Mobility Enhancement and Vision Rehabilitation Center of Excellence are working to find creative ways to help patients with impaired vision achieve greater independence and mobility, and a better quality of life. One vision-enhancing technology is SuperVision+, a free smart phone magnifier app available for iOS and Android platforms. In addition to magnifying small print (i.e., medication bottles and restaurant menus), the app has a unique image-stabilization feature that “locks” shaky images caused by hand tremors. Another tech-savvy application is utilizing video games to help patients develop navigation skills (way-finding) and improve their sense of independence. Center members are also involved in research addressing contrast sensitivity, fundus-related perimetry, and visual hallucinations in patients with vision loss, as well as development of a retinal prosthesis.
